New Draft Guidance to Support Risk-Based Computer Software Assurance. What about the Computer Software Assurance CSA guidance coming from the FDA.
Fda Computer Software Assurance Guidance. The FDA is preparing to release new guidance Computer Software Assurance for Manufacturing Operations and Quality Systems Software in late 2020. About the Author Darren Geaney is a Process Engineer with over 20 years experience in Quality Assurance specializing in Computer System Validation. Focus on critical thinking risk-based assurance needs testing activities and also documentation risk assessment will focus on the risk to. New Draft Guidance to Support Risk-Based Computer Software Assurance.
 Fda Move From Software Validation To Computer Software Assurance Csa From aodocs.com
Fda Move From Software Validation To Computer Software Assurance Csa From aodocs.com
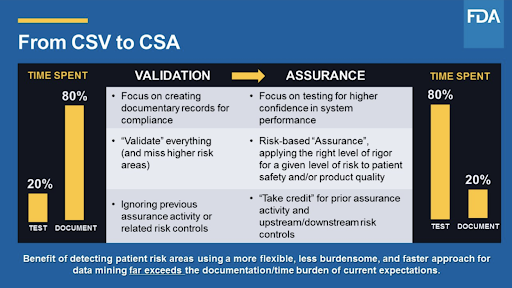
USFDA Center for Devices and Radiological Health CDRH draft guidance on Computer Software Assurance for Manufacturing Operations and Quality System Software. The FDA is expected to release its new guidance around CSA Computer Software Assurance for Manufacturing Operations and Quality Systems Software before the end of 2020. Focus on critical thinking risk-based assurance needs testing activities and also documentation risk assessment will focus on the risk to. This new guidance is highly anticipated because it will actually streamline some of your computer software systems. Computer Software Assurance Alliance CSA Alliance The intent of the Computer Software Assurance Alliance CSAA is to support and engage with the FDA Industry CSA team FICSA that creates and provides presentations videos case studies blogs and new examples to educate and promote the adoption of risk based CSA and critical best practices for the Life Sciences. The FDA draft guidance on Computer Software Assurance is a paradigm shift from document focused computer system validation to critical thinking assurance practices.
Focus on critical thinking risk-based assurance needs testing activities and also documentation risk assessment will focus on the risk to.
In 2019 FDA will be releasing a new draft guidance Computer Software Assurance for Manufacturing Operations and Quality System Software that updates 20 year legacy guidance documents found in 21 CFR Part 11 relating to medical device. Informational US FDA Final Guidance Coronary Peripheral and Neurovascular Guidewires Performance Tests and Recommended Labeling. USFDA Center for Devices and Radiological Health CDRH draft guidance on Computer Software Assurance for Manufacturing Operations and Quality System Software. Applied to any software.
 Source: amplelogic.com
Source: amplelogic.com

 Source: srutatechnologies.com
Source: srutatechnologies.com

The FDA draft guidance on Computer Software Assurance is a paradigm shift from document focused computer system validation to critical thinking assurance practices. This new guidance will provide guidelines for streamlining documentation with an emphasis on critical thinking risk management patient and product safety data integrity and quality assurance. New Draft Guidance to Support Risk-Based Computer Software Assurance. This new guidance is highly anticipated because it will actually streamline some of your computer software systems. What about the Computer Software Assurance CSA guidance coming from the FDA.

The FDAs general view of automation is basically a green light for companies. This document is intended to help industry understand the Computer System Validation CSV requirements of 21 CFR Part 11. The FDA announced that in September 2020 it will release guidance on Computer Software Assurance CSA. Computer Software Assurance for Manufacturing Operations and Quality System Software. Electronic Records and Signatures that was promulgated in 1997.

The FDAs general view of automation is basically a green light for companies. Informational US FDA Final Guidance Coronary Peripheral and Neurovascular Guidewires Performance Tests and Recommended Labeling. Computer Software Assurance CSA is a Case for Quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization and innovation. New Draft Guidance to Support Risk-Based Computer Software Assurance. Electronic Records and Signatures that was promulgated in 1997.
 Source: continuousvalidation.com
Source: continuousvalidation.com
Prioritized Guidance Documents that CDRH Intends to Publish in FY2021. Many people and organisations in the industry speculated regarding the way to decrease the needed compliance. Informational US FDA Draft Guidance Breast Implants Certain Labeling Recommendations to Improve Patient Communication. USFDA Center for Devices and Radiological Health CDRH draft guidance on Computer Software Assurance for Manufacturing Operations and Quality System Software. Computer Software Assurance for Manufacturing Operations and Quality System Software.
 Source: perfval.com
Source: perfval.com
A guidance topic currently in draft from the Center for Devices and Radiological Health CDRH titled Computer Software Assurance for Manufacturing Operations and Quality System Software aims to change the paradigm on how computer system validation is performed. Prioritized Guidance Documents that CDRH Intends to Publish in FY2021. Electronic Records and Signatures that was promulgated in 1997. The FDA is expected to release its new guidance around CSA Computer Software Assurance for Manufacturing Operations and Quality Systems Software before the end of 2020. A guidance topic currently in draft from the Center for Devices and Radiological Health CDRH titled Computer Software Assurance for Manufacturing Operations and Quality System Software aims to change the paradigm on how computer system validation is performed.
 Source: youtube.com
Source: youtube.com
Computer Software Assurance Alliance CSA Alliance The intent of the Computer Software Assurance Alliance CSAA is to support and engage with the FDA Industry CSA team FICSA that creates and provides presentations videos case studies blogs and new examples to educate and promote the adoption of risk based CSA and critical best practices for the Life Sciences. For FDA purposes this guidance applies to any software related to a regulated medical device as defined by Section 201h of the Federal Food Drug and Cosmetic Act. Applied to any software. This new guidance will provide guidelines for streamlining documentation with an emphasis on critical thinking risk management patient and product safety data integrity and quality assurance. Electronic Records and Signatures that was promulgated in 1997.
 Source: aodocs.com
Source: aodocs.com
A guidance topic currently in draft from the Center for Devices and Radiological Health CDRH titled Computer Software Assurance for Manufacturing Operations and Quality System Software aims to change the paradigm on how computer system validation is performed. To harmonize with international standards the FDAs Center for Devices and Radiological Health CDRH plans to release a new draft guidance Computer Software Assurance for Manufacturing Operations and Quality System Software that aligns with the current quality systems regulation ISO 13485. The Guidance is on FDAs list for release in September 2020 and applies to non-product quality system software. The FDA announced that in September 2020 it will release guidance on Computer Software Assurance CSA. The FDA is expected to release its new guidance around CSA Computer Software Assurance for Manufacturing Operations and Quality Systems Software before the end of 2020.
![]() Source: amplelogic.com
Source: amplelogic.com
USFDA Center for Devices and Radiological Health CDRH draft guidance on Computer Software Assurance for Manufacturing Operations and Quality System Software. USFDA Center for Devices and Radiological Health CDRH draft guidance on Computer Software Assurance for Manufacturing Operations and Quality System Software. Electronic Records and Signatures that was promulgated in 1997. Prioritized Guidance Documents that CDRH Intends to Publish in FY2021. The Guidance is on FDAs list for release in September 2020 and applies to non-product quality system software.
 Source: 3-14.com
Source: 3-14.com
To harmonize with international standards the FDAs Center for Devices and Radiological Health CDRH plans to release a new draft guidance Computer Software Assurance for Manufacturing Operations and Quality System Software that aligns with the current quality systems regulation ISO 13485. Computer Software Assurance for Manufacturing Operations and Quality System Software. Focus on critical thinking risk-based assurance needs testing activities and also documentation risk assessment will focus on the risk to. Prioritized Guidance Documents that CDRH Intends to Publish in FY2021. For FDA purposes this guidance applies to any software related to a regulated medical device as defined by Section 201h of the Federal Food Drug and Cosmetic Act.
 Source: srutatechnologies.com
Source: srutatechnologies.com
The FDAs general view of automation is basically a green light for companies. The FDA is preparing to release new guidance Computer Software Assurance for Manufacturing Operations and Quality Systems Software in late 2020. Informational US FDA Final Guidance Coronary Peripheral and Neurovascular Guidewires Performance Tests and Recommended Labeling. Applied to any software. The FDA is expected to release a new guidance document Computer Software Assurance for Manufacturing Operations and Quality System Software in 2021.

The FDA announced that in September 2020 it will release guidance on Computer Software Assurance CSA. The FDA is expected to release a new guidance document Computer Software Assurance for Manufacturing Operations and Quality System Software in 2021. FDA continues to encourage the use of innovative new technologies to support the development of quality new drugs and ensure that patient safety is uppermost in the development and manufacture of drugs. To harmonize with international standards the FDAs Center for Devices and Radiological Health CDRH plans to release a new draft guidance Computer Software Assurance for Manufacturing Operations and Quality System Software that aligns with the current quality systems regulation ISO 13485. Focus on critical thinking risk-based assurance needs testing activities and also documentation risk assessment will focus on the risk to.
 Source: slcontrols.com
Source: slcontrols.com
The FDA draft guidance on Computer Software Assurance is a paradigm shift from document focused computer system validation to critical thinking assurance practices. The FDA is leaning towards a Case for Quality CfQ approach with less emphasis on a compliance approach allowing device. This new guidance is highly anticipated because it will actually streamline some of your computer software systems. What about the Computer Software Assurance CSA guidance coming from the FDA. Computer Software Assurance Alliance CSA Alliance The intent of the Computer Software Assurance Alliance CSAA is to support and engage with the FDA Industry CSA team FICSA that creates and provides presentations videos case studies blogs and new examples to educate and promote the adoption of risk based CSA and critical best practices for the Life Sciences.

Prioritized Guidance Documents that CDRH Intends to Publish in FY2021. This document is intended to help industry understand the Computer System Validation CSV requirements of 21 CFR Part 11. Informational US FDA Final Guidance Coronary Peripheral and Neurovascular Guidewires Performance Tests and Recommended Labeling. FDA continues to encourage the use of innovative new technologies to support the development of quality new drugs and ensure that patient safety is uppermost in the development and manufacture of drugs. To harmonize with international standards the FDAs Center for Devices and Radiological Health CDRH plans to release a new draft guidance Computer Software Assurance for Manufacturing Operations and Quality System Software that aligns with the current quality systems regulation ISO 13485.

Computer Software Assurance for Manufacturing Operations and Quality System Software. Computer Software Assurance Alliance CSA Alliance The intent of the Computer Software Assurance Alliance CSAA is to support and engage with the FDA Industry CSA team FICSA that creates and provides presentations videos case studies blogs and new examples to educate and promote the adoption of risk based CSA and critical best practices for the Life Sciences. For FDA purposes this guidance applies to any software related to a regulated medical device as defined by Section 201h of the Federal Food Drug and Cosmetic Act. The FDAs general view of automation is basically a green light for companies. A guidance topic currently in draft from the Center for Devices and Radiological Health CDRH titled Computer Software Assurance for Manufacturing Operations and Quality System Software aims to change the paradigm on how computer system validation is performed.
 Source: srutatechnologies.com
Source: srutatechnologies.com
Computer Software Assurance for Manufacturing Operations and Quality System Software. USFDA Center for Devices and Radiological Health CDRH draft guidance on Computer Software Assurance for Manufacturing Operations and Quality System Software. The FDA is expected to release its new guidance around CSA Computer Software Assurance for Manufacturing Operations and Quality Systems Software before the end of 2020. The FDA draft guidance on Computer Software Assurance is a paradigm shift from document focused computer system validation to critical thinking assurance practices. Computer Software Assurance CSA is a Case for Quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization and innovation.