The common issues and resolutions related to assurance cases. To demonstrate safety to create and review a safety assurance case.
Fda Assurance Case. This part of ISOIEC 15026 specifies minimum requirements for the structure and contents of an assurance case. FDA Assurance Cases 21 22 Feb 2008 Based on Open Group Paris 23 April 2007 slight revisions of Open Group San Diego 31 January 2007 major rewrite of HCSS Aviation Safety Workshop Alexandria Oct 56 2006 Based on University of Illinois ITI Distinguished Lecture Wednesday 5 April 2006 based on ITCES invited talk Tuesday 4 April 2006. The FDAs guidance document titled Computer Software Assurance for Manufacturing and Quality System Software has its roots in a 2011 FDA study of the Case for Quality which examined the 2002 guidance document called Validation of Software in Medical Devices. FDA CSA Computer Software Assurance The Case for Quality.
 Computer Software Assurance Risk Assessment Takes Center Stage From perfval.com
Computer Software Assurance Risk Assessment Takes Center Stage From perfval.com
This part of ISOIEC 15026 specifies minimum requirements for the structure and contents of an assurance case. The infrastructure used must be qualified. Guidance development centered around assurance activities for this category. The FDA requires that a safety assurance case be included in 510 k submissions for infusion pumps. Wwwfdagov Case for Quality 6 Why Risk to patients from quality issues and hampered innovation in manufacturing and product development practices High industry focus on. The study revealed an inclination towards medical device software which is the product software not taking into.
The assurance case is a method for reasoning about systems appropriate for scientists and engineers.
This part of ISOIEC 15026 specifies minimum requirements for the structure and contents of an assurance case. It is always the result of high intention sincere effort intelligent direction and skillful execution. Guidance development centered around assurance activities for this category. And the structure templates and approach for using assurance cases to achieve three important goals.
 Source: ispe.org
Source: ispe.org
 Source: srutatechnologies.com
Source: srutatechnologies.com
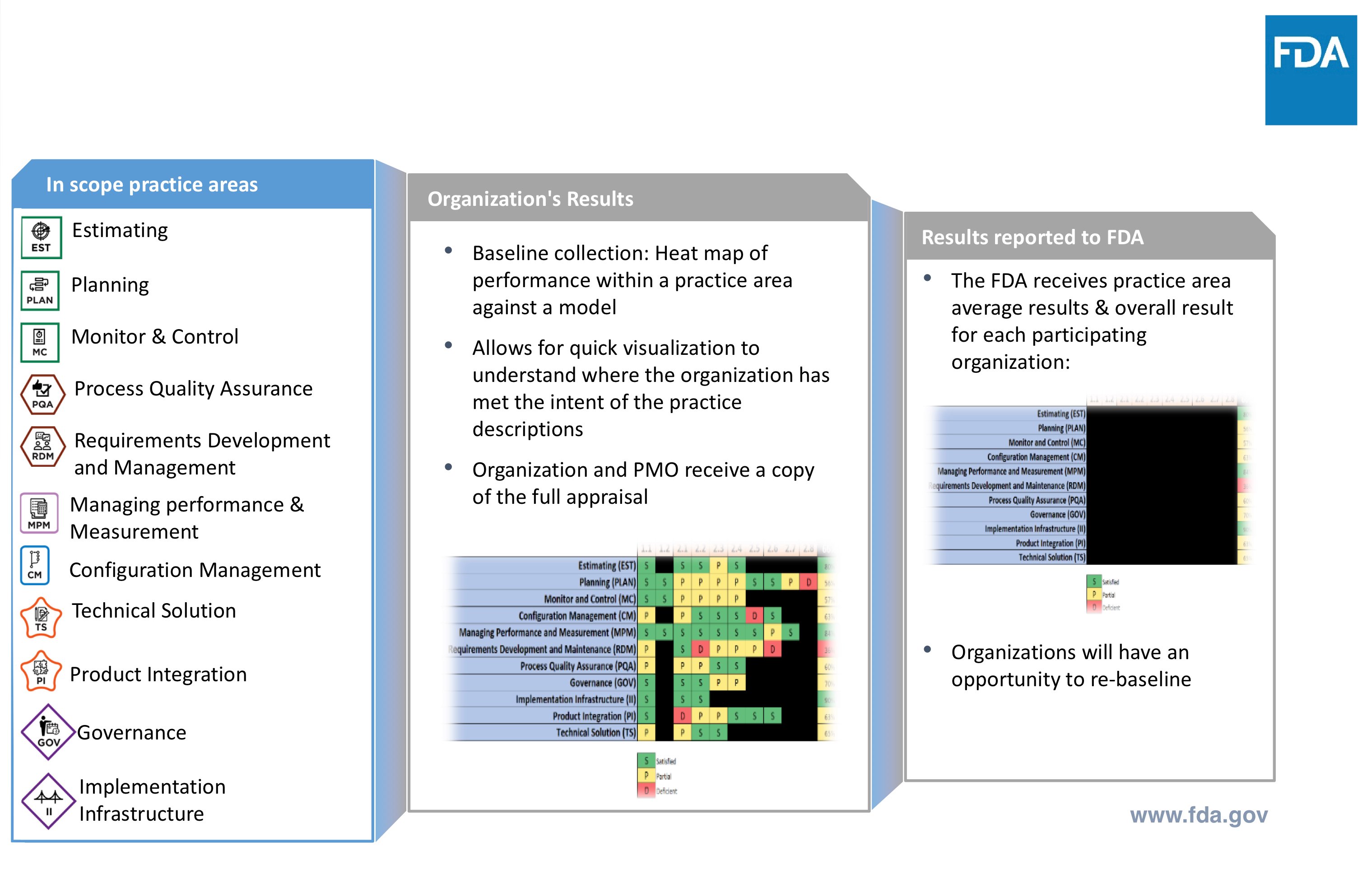 Source: greenlight.guru
Source: greenlight.guru
 Source: researchgate.net
Source: researchgate.net
A documented body of. Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA 1-888-463-6332. It covers the elements of assurance cases. The FDAs guidance document titled Computer Software Assurance for Manufacturing and Quality System Software has its roots in a 2011 FDA study of the Case for Quality which examined the 2002 guidance document called Validation of Software in Medical Devices. A safety case is a structured argument supported by a body of evidence that provides a compelling comprehensible and valid case that a system is safe for a given application in a given environment.
 Source: ispe.org
Source: ispe.org
That provides a convincing and valid. Warning Letter issued for the second site. Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA 1-888-463-6332. It represents the wise choice of many alternatives William A. Medical device safety assurance case guidance.
 Source: perfval.com
Source: perfval.com
It represents the wise choice of many alternatives William A. It is always the result of high intention sincere effort intelligent direction and skillful execution. It represents the wise choice of many alternatives William A. The study revealed an inclination towards medical device software which is the product software not taking into. The FDA requires that a safety assurance case be included in 510 k submissions for infusion pumps.
 Source: johner-institute.com
Source: johner-institute.com
This technical information report provides guidance on how to complete an Assurance Case Report in order to comply with the new additional FDA pre-market requirements for infusion pumps. The National Medical Device Curriculum is a set of four fictitious case studies intended to help academic institutions and medtech companies understand FDAs medical device regulatory processes. This technical information report provides guidance on how to complete an Assurance Case Report in order to comply with the new additional FDA pre-market requirements for infusion pumps. The study revealed an inclination towards medical device software which is the product software not taking into. The FDA initiated this pilot to gather information about industry concerns with safety assurance cases and how the FDA can provide more clarity on the development of safety assurance cases.

This 2-day course provides attendees with an understanding of safety assurance cases and how they can be applied to combination products to demonstrate safety and facilitate pre-market review communications with the FDA. The National Medical Device Curriculum is a set of four fictitious case studies intended to help academic institutions and medtech companies understand FDAs medical device regulatory processes. To demonstrate safety to create and review a safety assurance case. Regulations exist all over the world for the protection and safety of consumers. Computer Software Assurance CSA is a Case for Quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization and innovation.
 Source: researchgate.net
Source: researchgate.net
The infrastructure used must be qualified. The FDA believes that better understanding of regulatory processes will accelerate the delivery of innovative medical devices to. FDA launches four case studies to explain medical device regulation. FDA Assurance Cases 21 22 Feb 2008 Based on Open Group Paris 23 April 2007 slight revisions of Open Group San Diego 31 January 2007 major rewrite of HCSS Aviation Safety Workshop Alexandria Oct 56 2006 Based on University of Illinois ITI Distinguished Lecture Wednesday 5 April 2006 based on ITCES invited talk Tuesday 4 April 2006. Computer Software Assurance CSA is a Case for Quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization and innovation.
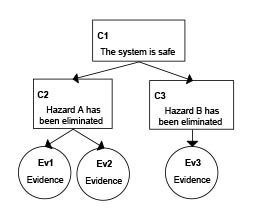 Source: insights.sei.cmu.edu
Source: insights.sei.cmu.edu
Safety Assurance Case and FDA. Wwwfdagov Non-Product CSV Modification Impact Activity Current Approach Modified Approach. That provides a convincing and valid. What Happened FDA placed both sites under import alert within two weeks of the second inspection. It covers the elements of assurance cases.

The FDA initiated this pilot to gather information about industry concerns with safety assurance cases and how the FDA can provide more clarity on the development of safety assurance cases. An assurance case addressing safety is called a safety case. In the regulated environment all computer-based systems used must be validated before they are put into productive use. FDA launches four case studies to explain medical device regulation. Wwwfdagov Case for Quality 6 Why Risk to patients from quality issues and hampered innovation in manufacturing and product development practices High industry focus on.
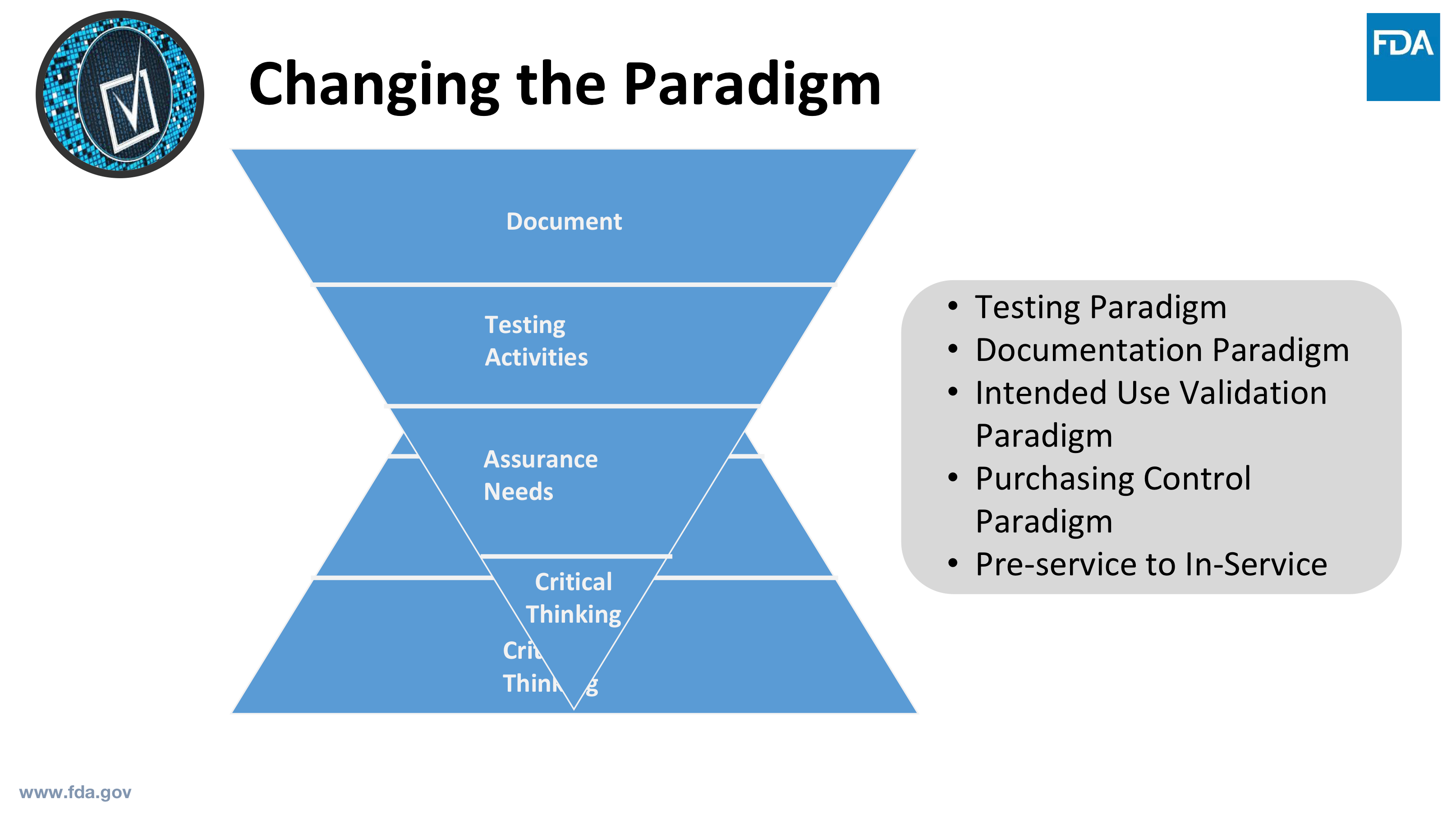 Source: greenlight.guru
Source: greenlight.guru
To demonstrate safety to create and review a safety assurance case. This technical information report provides guidance on how to complete an Assurance Case Report in order to comply with the new additional FDA pre-market requirements for infusion pumps. FDAs New Guidance for Software Assurance Quality is never an accident. An assurance case includes a top-level claim for a property of a system or product or set of claims systematic argumentation regarding this claim and the evidence and explicit assumptions that. FDA Assurance Cases 21 22 Feb 2008 Based on Open Group Paris 23 April 2007 slight revisions of Open Group San Diego 31 January 2007 major rewrite of HCSS Aviation Safety Workshop Alexandria Oct 56 2006 Based on University of Illinois ITI Distinguished Lecture Wednesday 5 April 2006 based on ITCES invited talk Tuesday 4 April 2006.

Wwwfdagov Case for Quality 6 Why Risk to patients from quality issues and hampered innovation in manufacturing and product development practices High industry focus on. In the regulated environment all computer-based systems used must be validated before they are put into productive use. To demonstrate safety to create and review a safety assurance case. The FDAs guidance document titled Computer Software Assurance for Manufacturing and Quality System Software has its roots in a 2011 FDA study of the Case for Quality which examined the 2002 guidance document called Validation of Software in Medical Devices. It includes a detailed but strictly hypothetical example from.
 Source: meddeviceonline.com
Source: meddeviceonline.com
Most of the combination products are drug delivery devices like infusion pumps and would be expected to have information like safety assurance cases be included in. In the regulated environment all computer-based systems used must be validated before they are put into productive use. A safety case is a structured argument supported by a body of evidence that provides a compelling comprehensible and valid case that a system is safe for a given application in a given environment. An assurance case includes a top-level claim for a property of a system or product or set of claims systematic argumentation regarding this claim and the evidence and explicit assumptions that. Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA 1-888-463-6332.

Warning Letter issued for the second site. Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA 1-888-463-6332. The FDA initiated this pilot to gather information about industry concerns with safety assurance cases and how the FDA can provide more clarity on the development of safety assurance cases. The study revealed an inclination towards medical device software which is the product software not taking into. The maintenance of the valid state must be ensured over the entire life cycle of the system.
 Source: researchgate.net
Source: researchgate.net
Regulations exist all over the world for the protection and safety of consumers. This 2-day course provides attendees with an understanding of safety assurance cases and how they can be applied to combination products to demonstrate safety and facilitate pre-market review communications with the FDA. That a specified set of. This technical information report provides guidance on how to complete an Assurance Case Report in order to comply with the new additional FDA pre-market requirements for infusion pumps. Warning Letter issued for the second site.
 Source: greenlight.guru
Source: greenlight.guru
This technical information report provides guidance on how to complete an Assurance Case Report in order to comply with the new additional FDA pre-market requirements for infusion pumps. Regulations exist all over the world for the protection and safety of consumers. FDA CSA Computer Software Assurance The Case for Quality. Understand how safety assurance case can help to address limitations with common risk management methods practices Ask FDA questions about risk management and safety assurance cases FDA participants are available for questions after the session content has been presented. FDA Assurance Cases 21 22 Feb 2008 Based on Open Group Paris 23 April 2007 slight revisions of Open Group San Diego 31 January 2007 major rewrite of HCSS Aviation Safety Workshop Alexandria Oct 56 2006 Based on University of Illinois ITI Distinguished Lecture Wednesday 5 April 2006 based on ITCES invited talk Tuesday 4 April 2006.
 Source: researchgate.net
Source: researchgate.net
The study revealed an inclination towards medical device software which is the product software not taking into. An assurance case includes a top-level claim for a property of a system or product or set of claims systematic argumentation regarding this claim and the evidence and explicit assumptions that. FDAs New Guidance for Software Assurance Quality is never an accident. Warning Letter issued for the second site. Wwwfdagov Case for Quality 6 Why Risk to patients from quality issues and hampered innovation in manufacturing and product development practices High industry focus on.





