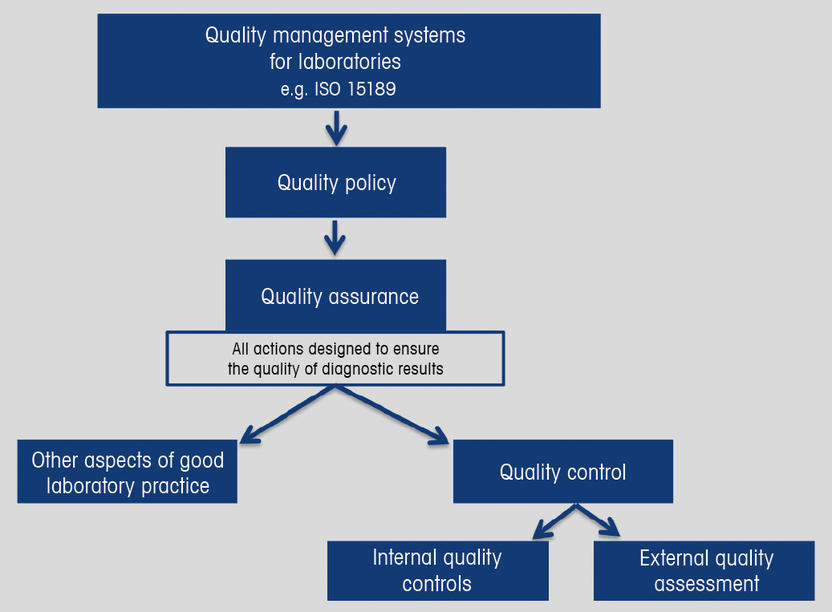
Within hospital laboratories two of the essential components of the quality system are quality assurance and quality control. Quality control is focused on fulfilling quality requirements and as related to clinical trials it encompasses the operational techniques and activities undertaken within the quality assurance system to verify that the requirements for quality of the trial-related activities have been fulfilled.
Definition Of Quality Assurance In Medical Laboratory. Examples from which errors emanates. Definition of quality Laboratory quality can be defined as accuracy reliability and timeliness of the reported test results. In the laboratory the instruments and methodologies must be monitored to ensure accurate results. Quality assurance QA is the total process whereby the quality of laboratory reports can be guaranteed.
 Four Types Of Quality Control Video Lesson Transcript Study Com From study.com
Four Types Of Quality Control Video Lesson Transcript Study Com From study.com
In order to control the quality of the procedures these programs are a tool to keep the laboratory procedures and every variable involved in staff equipment and method well controlled. Examples from which errors emanates. Pre-analytical analytical testing and post-analytical processes. Quality assurance is the monitoring of any activity that is associated with a laboratory result. A reflection on quality assurance and its application in hospital laboratories and in the community POCT to indicate competence in understanding the importance in a health care setting. Documentation Standard Operating Procedures SOPs Quality Control samples.
Pre-analytical analytical testing and post-analytical processes.
The laboratory results must be as accurate as possible all aspects of the laboratory operations must be reliable and reporting must be timely in order to. Definition rationale brief statement describing supporting health-related reasons quality gap AHRQ health importance and potential for improving health and evidence base AHRQ scientific soundnessclinical logic criteria associated with quality of care outcomes and. External quality assurance EQA external quality assessment EQA proficiency testing scheme System designed to objectively assess the quality of results obtained by laboratories by means of an external agency. Summarized information for each of the 14 reviewed laboratory medicine quality indicators is provided in the following format.
 Source: slideplayer.com
Source: slideplayer.com
 Source: youtube.com
Source: youtube.com
 Source: nap.edu
Source: nap.edu
 Source: study.com
Source: study.com
Are procedures used in each assay to assure a test run is valid and results are reliable. Summarized information for each of the 14 reviewed laboratory medicine quality indicators is provided in the following format. Within hospital laboratories two of the essential components of the quality system are quality assurance and quality control. Quality ControlQuality Assurance QCQA can be defined as the set of planned and systematic activities focused on providing confidence that quality requirements will be fulfilled. Definition rationale brief statement describing supporting health-related reasons quality gap AHRQ health importance and potential for improving health and evidence base AHRQ scientific soundnessclinical logic criteria associated with quality of care outcomes and.
 Source: slideshare.net
Source: slideshare.net
All materials equipment and procedur. Summarized information for each of the 14 reviewed laboratory medicine quality indicators is provided in the following format. In order to control the quality of the procedures these programs are a tool to keep the laboratory procedures and every variable involved in staff equipment and method well controlled. A reflection on quality assurance and its application in hospital laboratories and in the community POCT to indicate competence in understanding the importance in a health care setting. Farlex Partner Medical Dictionary Farlex 2012.
 Source: slideplayer.com
Source: slideplayer.com
In the laboratory the instruments and methodologies must be monitored to ensure accurate results. Definition rationale brief statement describing supporting health-related reasons quality gap AHRQ health importance and potential for improving health and evidence base AHRQ scientific soundnessclinical logic criteria associated with quality of care outcomes and. In the laboratory the instruments and methodologies must be monitored to ensure accurate results. In the laboratory quality assurance involves the entire testing process. Within hospital laboratories two of the essential components of the quality system are quality assurance and quality control.
 Source: slideserve.com
Source: slideserve.com
A reflection on quality assurance and its application in hospital laboratories and in the community POCT to indicate competence in understanding the importance in a health care setting. All materials equipment and procedur. Quality ControlQuality Assurance. Quality ControlQuality Assurance QCQA can be defined as the set of planned and systematic activities focused on providing confidence that quality requirements will be fulfilled. Quality control is focused on fulfilling quality requirements and as related to clinical trials it encompasses the operational techniques and activities undertaken within the quality assurance system to verify that the requirements for quality of the trial-related activities have been fulfilled.
 Source: study.com
Source: study.com
Within hospital laboratories two of the essential components of the quality system are quality assurance and quality control. Farlex Partner Medical Dictionary Farlex 2012. Examples from which errors emanates. An audit is a quality improvement process and is an essential part of the quality assurance programme of a laboratory. It covers a wide range of matters that influence the quality of a product or service.
 Source: slideshare.net
Source: slideshare.net
Definition of quality Laboratory quality can be defined as accuracy reliability and timeliness of the reported test results. Farlex Partner Medical Dictionary Farlex 2012. And influence 70 of medical diagnoses1 Audit means to evaluate and in the context of the pathology laboratory would mean a systematic and critical analysis of pathology services. The standard definition of. Are procedures used in each assay to assure a test run is valid and results are reliable.
 Source: q-more.chemeurope.com
Source: q-more.chemeurope.com
In order to control the quality of the procedures these programs are a tool to keep the laboratory procedures and every variable involved in staff equipment and method well controlled. Quality control is focused on fulfilling quality requirements and as related to clinical trials it encompasses the operational techniques and activities undertaken within the quality assurance system to verify that the requirements for quality of the trial-related activities have been fulfilled. Wrong test tube wrong test ordered insufficient amount of specimen. Kit Controls Quality Control Samples. External quality assurance EQA external quality assessment EQA proficiency testing scheme System designed to objectively assess the quality of results obtained by laboratories by means of an external agency.
 Source: labpedia.net
Source: labpedia.net
In a medical laboratory. Examples from which errors emanates. Are procedures used in each assay to assure a test run is valid and results are reliable. Within hospital laboratories two of the essential components of the quality system are quality assurance and quality control. Wrong test tube wrong test ordered insufficient amount of specimen.
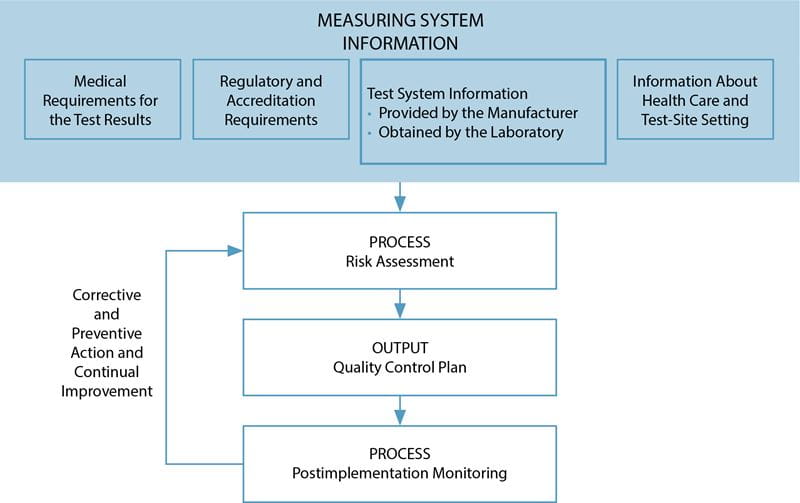 Source: acutecaretesting.org
Source: acutecaretesting.org
Examples from which errors emanates. Definition rationale brief statement describing supporting health-related reasons quality gap AHRQ health importance and potential for improving health and evidence base AHRQ scientific soundnessclinical logic criteria associated with quality of care outcomes and. Are procedures used in each assay to assure a test run is valid and results are reliable. Definition of quality Laboratory quality can be defined as accuracy reliability and timeliness of the reported test results. Quality ControlQuality Assurance.
 Source: slidetodoc.com
Source: slidetodoc.com
Definition rationale brief statement describing supporting health-related reasons quality gap AHRQ health importance and potential for improving health and evidence base AHRQ scientific soundnessclinical logic criteria associated with quality of care outcomes and. Summarized information for each of the 14 reviewed laboratory medicine quality indicators is provided in the following format. The laboratory results must be as accurate as possible all aspects of the laboratory operations must be reliable and reporting must be timely in order to. Quality ControlQuality Assurance QCQA can be defined as the set of planned and systematic activities focused on providing confidence that quality requirements will be fulfilled. Quality control is focused on fulfilling quality requirements and as related to clinical trials it encompasses the operational techniques and activities undertaken within the quality assurance system to verify that the requirements for quality of the trial-related activities have been fulfilled.
 Source: slideshare.net
Source: slideshare.net
Quality assurance is the monitoring of any activity that is associated with a laboratory result. Quality ControlQuality Assurance. Pre-analytical analytical testing and post-analytical processes. The standard definition of. Quality ControlQuality Assurance QCQA can be defined as the set of planned and systematic activities focused on providing confidence that quality requirements will be fulfilled.
 Source: nap.edu
Source: nap.edu
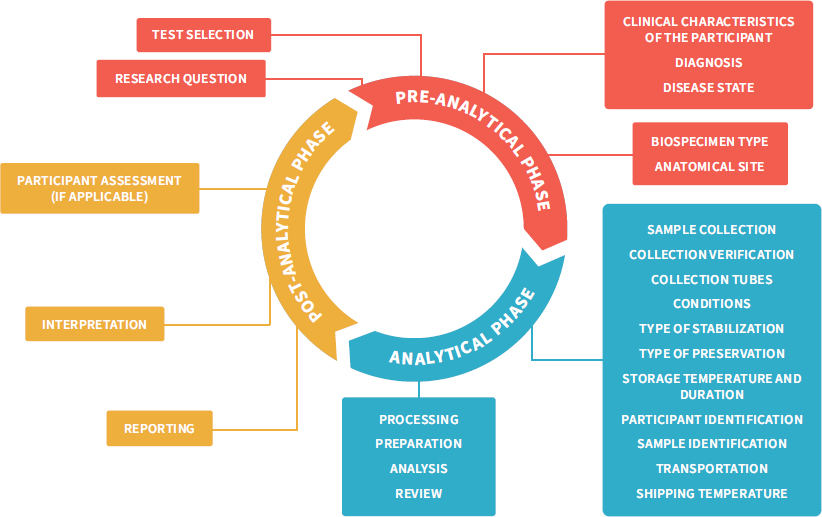
Summarized information for each of the 14 reviewed laboratory medicine quality indicators is provided in the following format. Quality ControlQuality Assurance. Quality assurance QA is the total process whereby the quality of laboratory reports can be guaranteed. Kit Controls Quality Control Samples. Quality Assurance in the Pre-analytical Phase.
 Source: slideplayer.com
Source: slideplayer.com
Kwahli-tē a-shūrănts Programs of regular assessment of medical and nursing activities to evaluate the quality of medical care. Within hospital laboratories two of the essential components of the quality system are quality assurance and quality control. The standard definition of. In the frame of a quality management system benefits from external quality assurance programs are discussed and different available designs are reviewed. Kit Controls Quality Control Samples.
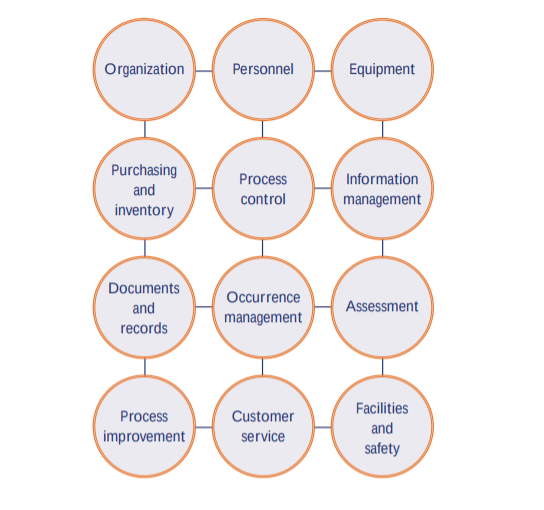 Source: qualio.com
Source: qualio.com
External quality assurance EQA external quality assessment EQA proficiency testing scheme System designed to objectively assess the quality of results obtained by laboratories by means of an external agency. It has been estimated that up to 30 of laboratory tests ordered each month are repeat tests. In the frame of a quality management system benefits from external quality assurance programs are discussed and different available designs are reviewed. All materials equipment and procedur. Laboratory Quality Assurance QA encompasses a range of activities that enable laboratories to achieve and maintain high levels of accuracy and proficiency despite changes in test methods and the volume of specimens tested.
 Source: researchgate.net
Source: researchgate.net
Quality Assurance in the Pre-analytical Phase. Within hospital laboratories two of the essential components of the quality system are quality assurance and quality control. The standard definition of. In the laboratory quality assurance involves the entire testing process. It covers a wide range of matters that influence the quality of a product or service.





