Request a Certificate of Quality Assurance Use this form to request a certificate of quality assurance from COPAN. For CBD a Certificate of Analysis COA should contain the contents of the product including total cannabinoids microbiological levels terpenes and possible contaminants such as pesticides solvent residue or heavy metals.
Coa Quality Assurance. COA Certificate of analysis or SGS report available for all our products. The C of A is part of the manufacturing quality assurance documentation. COAs are defined as documents issued by Quality Assurance that confirms a regulated product meets its product specification. Shortcuts for power users - examples.
 Pengertian Certificate Of Analysist Dan Kegunaanya Mister Exportir From misterexportir.com
Pengertian Certificate Of Analysist Dan Kegunaanya Mister Exportir From misterexportir.com
COAs are defined as documents issued by Quality Assurance that confirm a regulated product meets its product specification. Coke has always been keen to maintain its good brand quality so we lead food safety and supplier strategy from the center Marshall said. Komponen yang menunjang untuk mencapai mutu obat yang bagus divisi control meliputi. 11 Quality Assurance Review Handbook QUA ASSURANCE IN FINANCIAL AUDITING Table 1. Certificate of Analysis COA On the other hand a Certificate of Analysis COA is a document normally issued by Quality Assurance that authenticates that a regulated product fulfills its product requirements. COA means Construction Quality Assurance.
Get in Touch Contact Copan Get More Information Our Promise COPAN will continue to invest in science and technology to continually improve its knowledge to offer the most innovative and technically diverse product line in its niche.
Search for abbreviation meaning word to abbreviate or category. COAs are defined as documents issued by Quality Assurance that confirm a regulated product meets its product specification. The format and organization of the information on the CoA is at the issuing laboratorys discretion. The items included are based on WHO good practices for pharmaceutical quality control laboratories and.
 Source: qualityessentialssuite.com
Source: qualityessentialssuite.com
 Source: coavainc.com
Source: coavainc.com
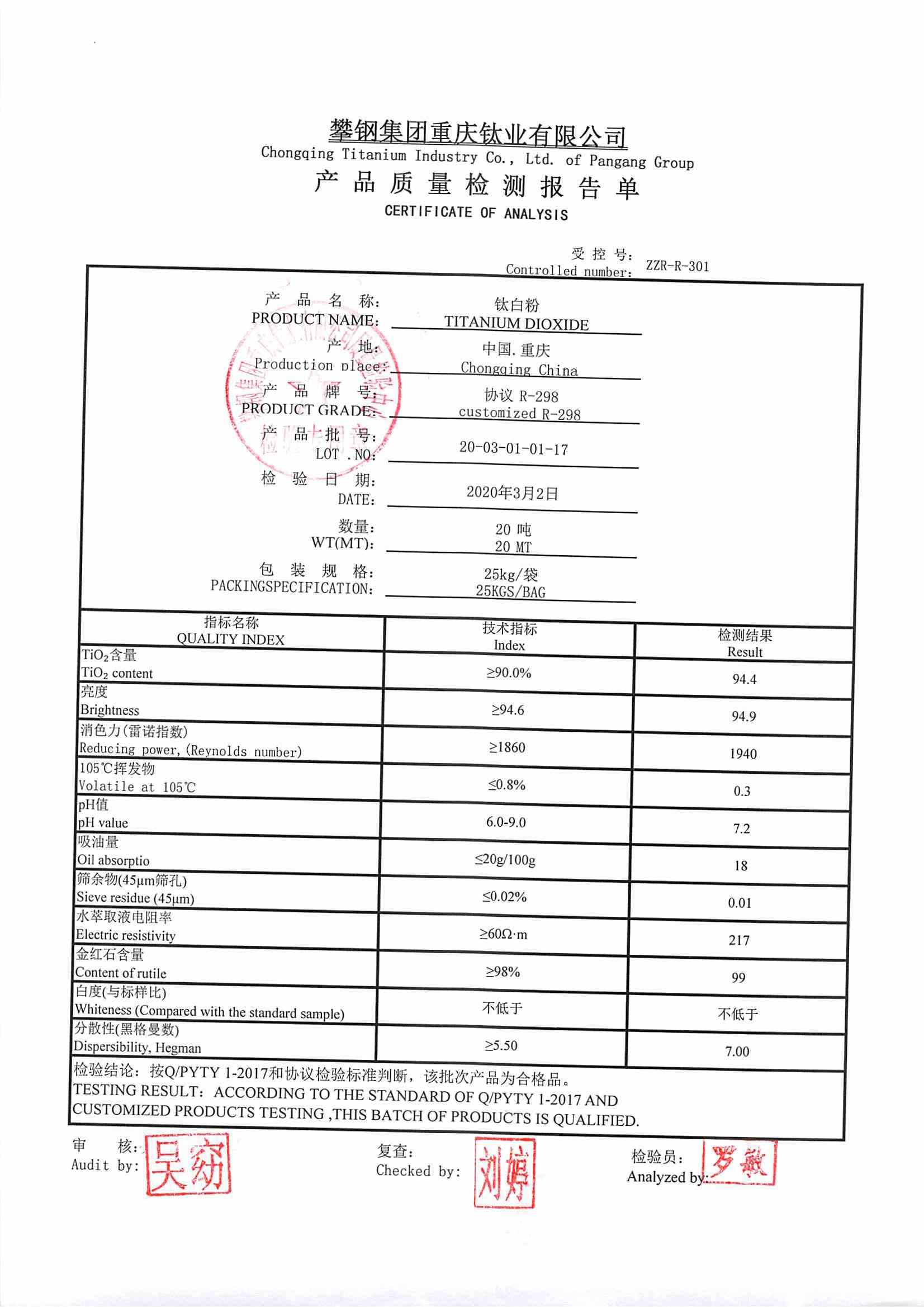 Source: cctio2.com
Source: cctio2.com
 Source: yumpu.com
Source: yumpu.com
The CoA can be printed on letterhead with the logo of the issuing laboratory. According to WHO good practices for pharmaceutical quality control laboratories 2 the CoA lists tests performed on a. You never know what youre going to get unless you do a little reading. COA is an abbreviation for Construction Quality Assurance. Quality Control QC.
 Source: cctio2.com
Source: cctio2.com
COA is an abbreviation for Construction Quality Assurance. To abbreviate - Management abbreviated. Amazon have separated C of A out from GMP I think which is odd as a C of A is part of a GMP system you can have certified and non certified GMP systems many companies have in-house quality systems but dont get them validated by ISO or another body. CBD is like a box of chocolates. Search for abbreviation meaning word to abbreviate or category.
 Source: acronymsandslang.com
Source: acronymsandslang.com
Our CoA system delivers in 3 distinct ways. The items included are based on WHO good practices for pharmaceutical quality control laboratories and. To abbreviate - Management abbreviated. COA Certificate of analysis or SGS report available for all our products. COAs are defined as documents issued by Quality Assurance that confirm a regulated product meets its product specification.
 Source: coavainc.com
Source: coavainc.com
11 Quality Assurance Review Handbook QUA ASSURANCE IN FINANCIAL AUDITING Table 1. Get in Touch Contact Copan Get More Information Our Promise COPAN will continue to invest in science and technology to continually improve its knowledge to offer the most innovative and technically diverse product line in its niche. Quality Control QC. Our CoA system delivers in 3 distinct ways. COA is an abbreviation for Construction Quality Assurance.
 Source: cctio2.com
Source: cctio2.com
Please ask us for COA or SGS report if you like. COA or SGS for product quality assurance. COAs are defined as documents issued by Quality Assurance that confirms a regulated product meets its product specification. COA Certificate of analysis or SGS report available for all our products. Request a Certificate of Quality Assurance Use this form to request a certificate of quality assurance from COPAN.
 Source: fitish.com
Source: fitish.com
The items included are based on WHO good practices for pharmaceutical quality control laboratories and. Coke has always been keen to maintain its good brand quality so we lead food safety and supplier strategy from the center Marshall said. 11 Quality Assurance Review Handbook QUA ASSURANCE IN FINANCIAL AUDITING Table 1. Search for abbreviation meaning word to abbreviate or category. A COA is as much for the customer as it is for the company to double-check the quality of their work for quality assurance purposes.
 Source: qualityessentialssuite.com
Source: qualityessentialssuite.com
CBD is like a box of chocolates. COA means Construction Quality Assurance. Quality Assurance QA jaminan mutu. Quality Control QC. They commonly contain the actual results obtained from testing performed as part of quality control of an individual batch of a product.
 Source: certificateof.com
Source: certificateof.com
The CoA can be printed on letterhead with the logo of the issuing laboratory. COA means Construction Quality Assurance. COAs are defined as documents issued by Quality Assurance that confirms a regulated product meets its product specification. The format and organization of the information on the CoA is at the issuing laboratorys discretion. Komponen yang menunjang untuk mencapai mutu obat yang bagus divisi control meliputi.
 Source: cctio2.com
Source: cctio2.com
The items included are based on WHO good practices for pharmaceutical quality control laboratories and. Certificate of Analysis COA A certificate of analysis COA is the suppliers test results on the specific lot being provided to you. Quality Assurance QA Adalah suatu konsep yang luas yang mencakup semua aspek yang secara kolektif maupun individual mempengaruhi mutu dari konsep design hingga product tersebut ditangan konsumen. The C of A is part of the manufacturing quality assurance documentation. This is a quality assurance document that indicates that a regulated product contains what it says it contains and meets product specifications.
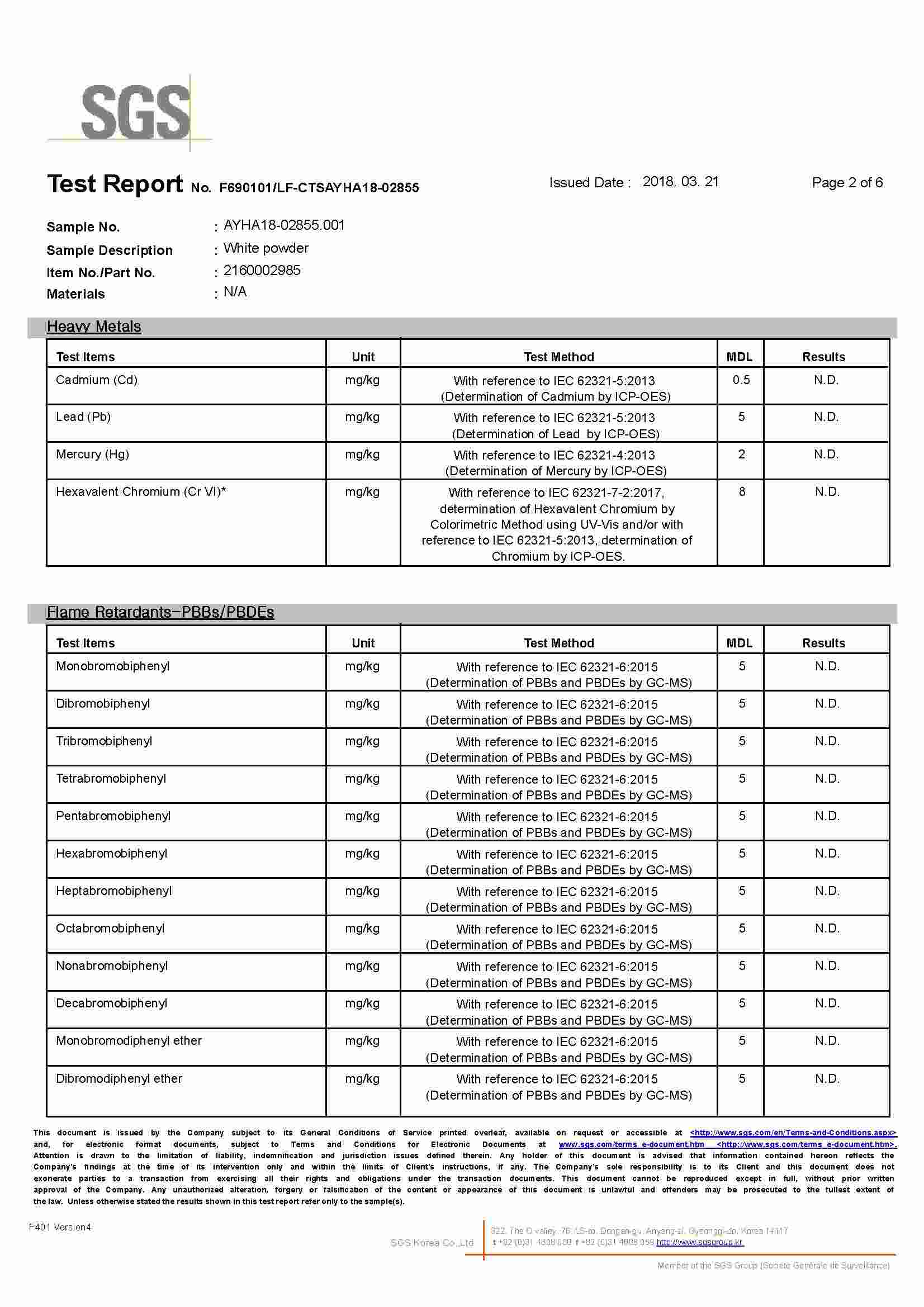 Source: cctio2.com
Source: cctio2.com
Request a Certificate of Quality Assurance Use this form to request a certificate of quality assurance from COPAN. A COA is as much for the customer as it is for the company to double-check the quality of their work for quality assurance purposes. The C of A is part of the manufacturing quality assurance documentation. According to WHO good practices for pharmaceutical quality control laboratories 2 the CoA lists tests performed on a. This is a quality assurance document that indicates that a regulated product contains what it says it contains and meets product specifications.
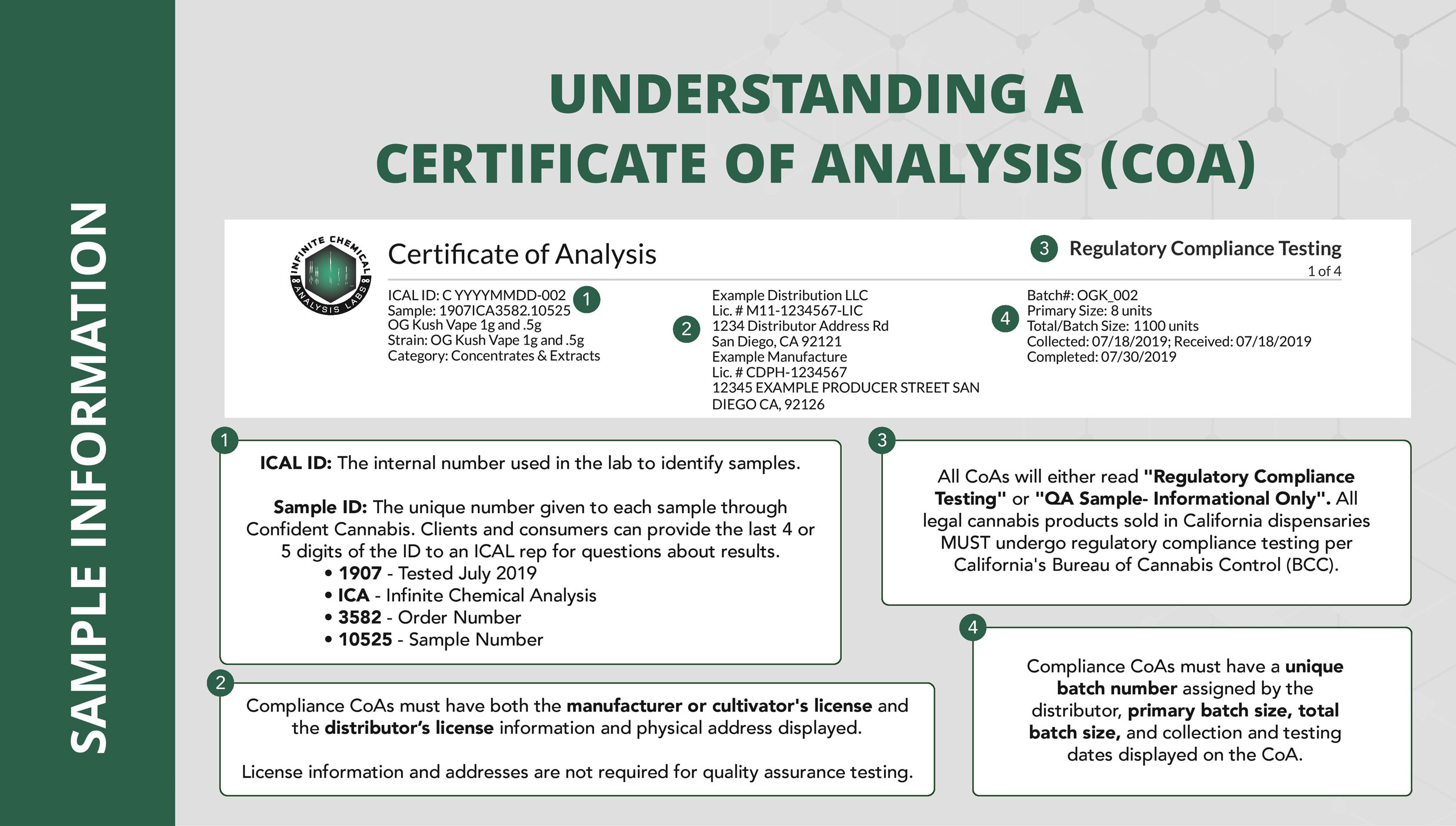 Source: infinitecal.com
Source: infinitecal.com
Request a Certificate of Quality Assurance Use this form to request a certificate of quality assurance from COPAN. A COA is as much for the customer as it is for the company to double-check the quality of their work for quality assurance purposes. Certificates of analysis COAs hold the key to improving quality and productivity and focusing on continuous improvement rather than just covering your assets. Search for abbreviation meaning word to abbreviate or category. General Requirements It has been recommended in various forums that the World Health Organization WHO should establish a model certificate of analysis CoA for use by quality control laboratories and in trade in starting materials and finished pharmaceutical products FPPs.
 Source: sclabs.com
Source: sclabs.com
Komponen yang menunjang untuk mencapai mutu obat yang bagus divisi control meliputi. COA means Construction Quality Assurance. CBD is like a box of chocolates. You never know what youre going to get unless you do a little reading. The importance of Certificates of Analysis is increasing especially during the event of outsourcing and globalization.
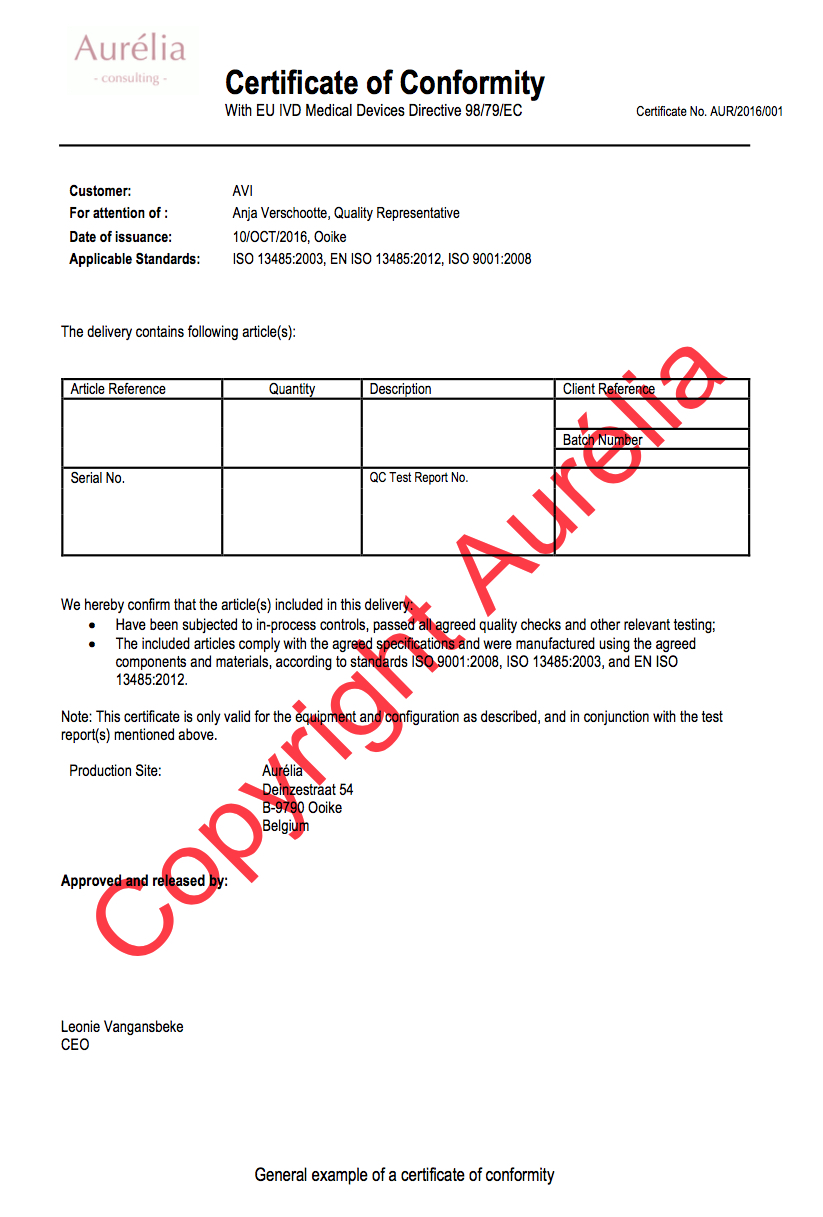 Source: aureliaconsulting.be
Source: aureliaconsulting.be
COAs are defined as documents issued by Quality Assurance that confirms a regulated product meets its product specification. The CoA can be printed on letterhead with the logo of the issuing laboratory. You never know what youre going to get unless you do a little reading. Certificate of Analysis COA On the other hand a Certificate of Analysis COA is a document normally issued by Quality Assurance that authenticates that a regulated product fulfills its product requirements. Search for abbreviation meaning word to abbreviate or category.
 Source: loket.com
Source: loket.com
The items included are based on WHO good practices for pharmaceutical quality control laboratories and. The importance of Certificates of Analysis is increasing especially during the event of outsourcing and globalization. Please ask us for COA or SGS report if you like. To abbreviate - Management abbreviated. General Requirements It has been recommended in various forums that the World Health Organization WHO should establish a model certificate of analysis CoA for use by quality control laboratories and in trade in starting materials and finished pharmaceutical products FPPs.
 Source: qualityessentialssuite.com
Source: qualityessentialssuite.com
Shortcuts for power users - examples. With CBD oil it should be as easy as. 11 Quality Assurance Review Handbook QUA ASSURANCE IN FINANCIAL AUDITING Table 1. Please ask us for COA or SGS report if you like. Desired Conditions for the Eight Elements of the COA-QMS Element of Framework Desired Condition Independence and Legal Framework The COA should maintain its independence and perform its mandate as provided for under the 1987 Constitution and Presidential Decree.





